Orfenix led the reformulation of cysteamine, partnering to repurpose the drug and provide clinical support for treating cystinosis, a rare disorder affecting children.

Orfenix initiated the development of an reformulated cysteamine by bringing key partners together in order to find an alternative development pathway in drug repurposing. Orfenix provides the clinical and regulatory support for Patient One and its mission to develop a product that is effectively available to patients suffering from cystinosis, a rare disorder that often appears in very young children and causes kidney failure, vomiting, and can affect patients’ eyes.
Patient One works to make living with cystinosis more bearable. Cystinosis is a rare, genetic metabolic disorder that affects the body’s ability to transport the amino acid cystine out of cells. A defective carrier protein leads to a high concentration of cystine in the cell organelles called lysosomes. As cystine is highly insoluble, this accumulation leads to the formation of crystals in almost all organs and tissues. The resulting cystine crystals can cause severe damage to organs, especially the kidneys and eyes. This condition is chronic and requires life-long management, making it a burden for patients and their families.
Currently, the most prescribed treatment for cystinosis is Cystagon. However, administering Cystagon comes with its own set of challenges for patients. It requires taking the medication four times daily, which can be a significant burden on patients’ daily routines, potentially leading to adherence and compliance issues. Cystagon has been on the market since 1997, however, as Cystinosis is a so called orphan disease, there has been little new innovation. Procysbi, an alternative medication that allows for twice-daily dosing, represents a promising step towards alleviating the burden on patients. However, despite its potential benefits, Procysbi is not widely accessible to patients due to its prohibitively high price, effectively excluding many who would benefit from this treatment regimen.
At Patient One, we have reformulated cysteamine. This novel formulation offers a more manageable treatment solution for cystinosis patients, allowing for twice-daily dosing, while ensuring affordability for those in need. By repurposing the existing product, working closely with academic partners we leverage each other’s strengths.
Orfenix’s expertise around orphan drug development provides Patient One with clinical support, regulatory support, and protocol support. In addition, our commitment to Socially responsible licensing ensures we work to make this treatment effectively available to patients. This sets us apart and illustrates how we make a meaningful difference in the lives of cystinosis patients worldwide.
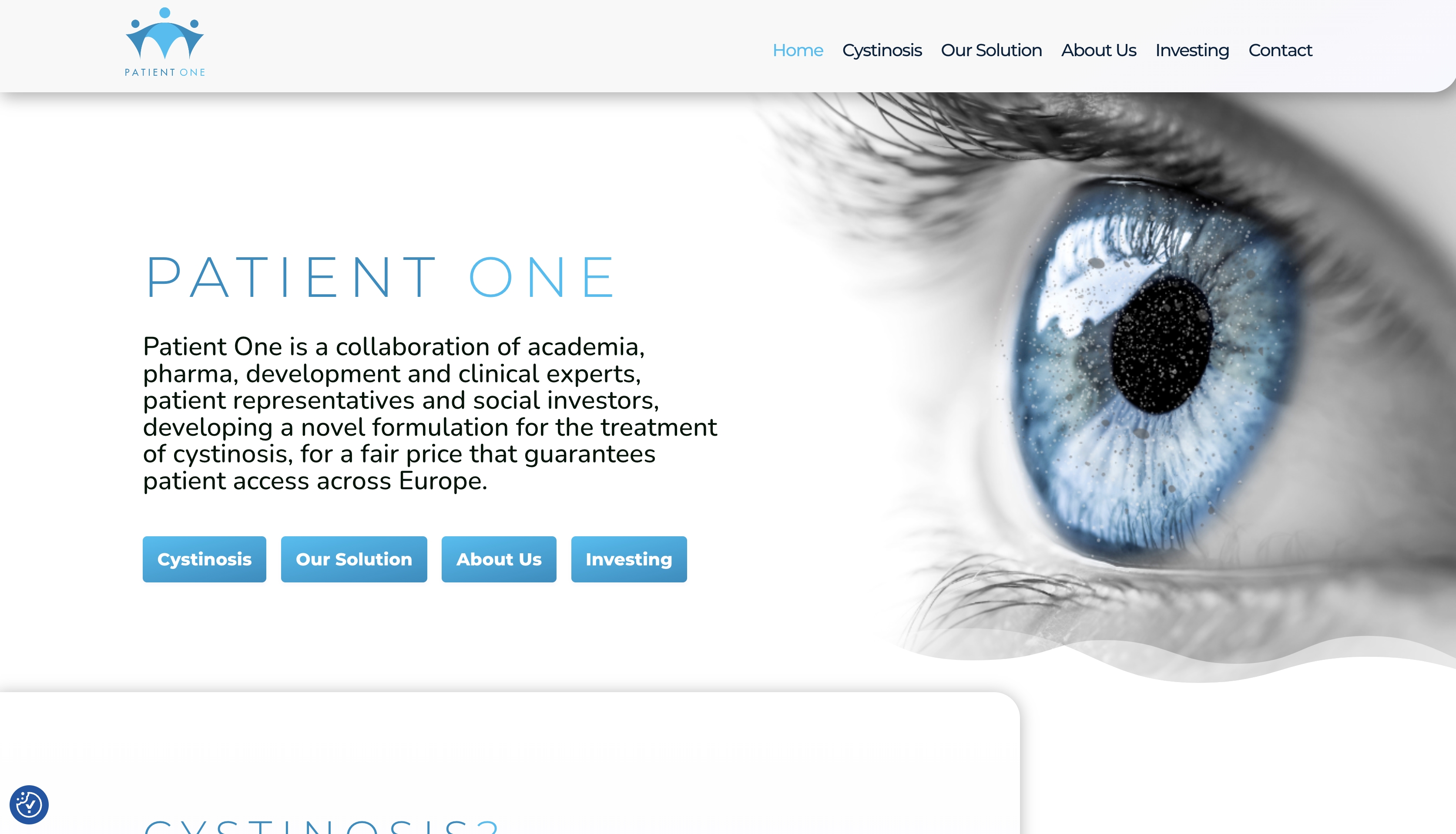
PATIENT ONE
Patient One is a collaboration of academia, pharma, development and clinical experts, patient representatives and social investors, developing a novel formulation for the treatment of cystinosis, for a fair price that guarantees patient access across Europe.
→ Explore more on the website